Explore New Options in 2026
Pharmaceutical marketers face a changing landscape. The traditional one‑size‑fits‑all campaign model no longer meets expectations. Patients and health‑care professionals (HCPs) are accustomed to personalized digital experiences in retail and expect the same seamless journey in health care. Yet the health sector presents unique challenges: data silos, complex care pathways, and strict privacy regulations make it difficult to deliver relevant, timely information.
At the same time, the rise of artificial intelligence (AI) and the explosion of health‑care data offer unprecedented opportunities.
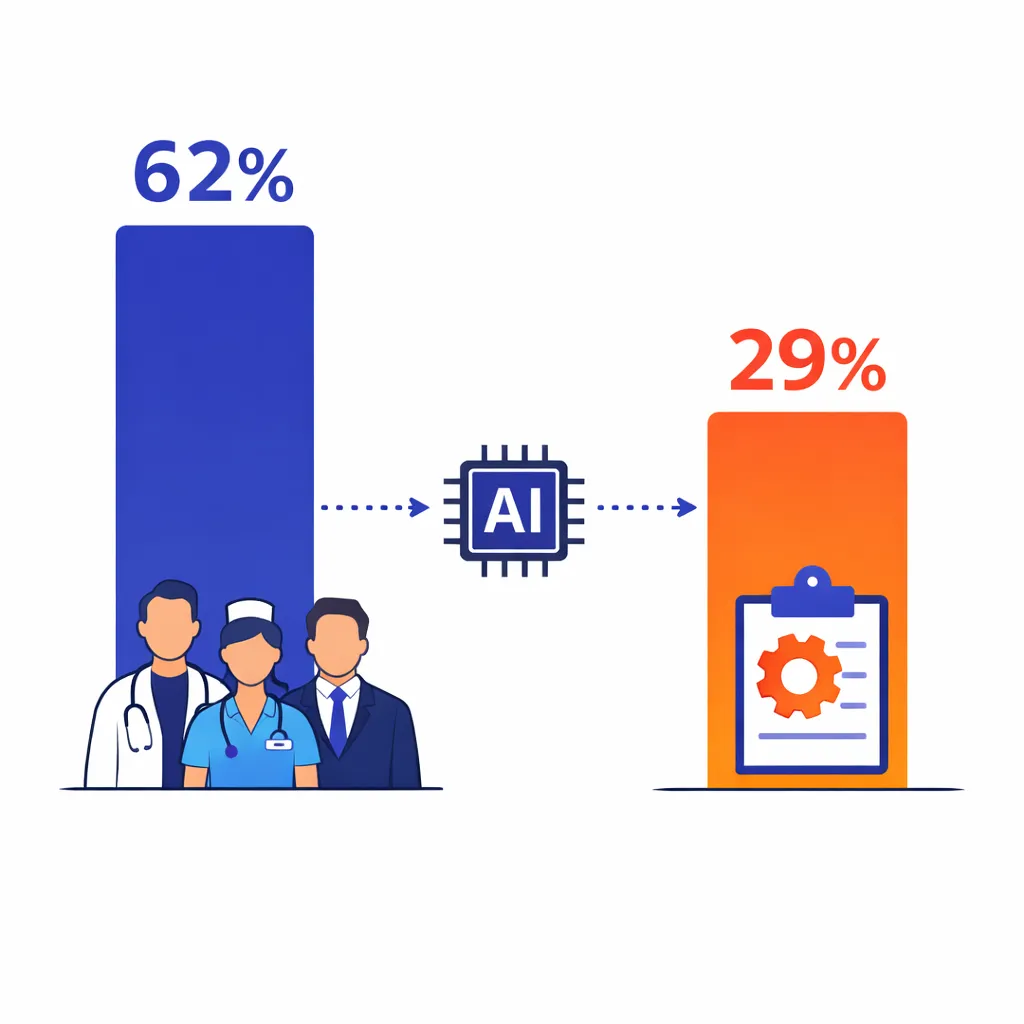
According to a 2024 McKinsey survey, 62 percent of health‑care leaders see consumer engagement and experience as the area where generative AI can make the greatest impact[1], but only 29 percent have begun implementing it[1].
This gap suggests a competitive advantage for early adopters.
This article explores how pharmaceutical marketers can personalize patient journeys using AI‑driven customer relationship management (CRM). We will cover the regulatory context, the science of patient journey mapping, the features of an AI‑enabled health‑care CRM, common misconceptions, and practical steps to get started. Throughout, we will refer to authoritative sources — government guidance on privacy, peer‑reviewed research on patient journey mapping and medication adherence, and industry analysis on AI’s potential. Our goal is to help marketers deliver relevant information that supports patients and HCPs while remaining compliant and ethical.
Why the Patient Journey Matters
A patient journey describes the stages a person experiences from awareness of a health issue to ongoing management or resolution. Mapping this journey helps marketers understand emotions, pain points, and information needs at each touchpoint. A 2025 scoping review published in the Journal of Diabetes Science and Technology notes that journey mapping (JM) and service blueprinting (SB) are valuable tools for visualizing user experiences and system processes over time[2].
The review found that most digital health behaviour change innovations used JMs to identify relevant touchpoints across the patient journey[3] and involved patients directly in the design process, treating JMs as sensemaking tools[4]. Importantly, JMs highlight opportunities for tailored support and reveal gaps where patients may drop out or feel unsupported[5].
Pharmaceutical marketers can use patient journey mapping to:

Identify touchpoints and pain points:
JMs break journeys into stages (e.g., awareness, diagnosis, treatment initiation, adherence, maintenance)[6].
At each stage, marketers can note questions patients ask, barriers they encounter, and preferred communication channels.
Co‑create solutions with patients:
The scoping review emphasised that patients frequently participated in JM processes[4].
Engaging patients helps ensure communications resonate and avoid paternalistic messaging.

Prioritize behavioral interventions:
JMs often depict actions, emotions, and system interactions[7], allowing marketers to focus resources on moments that influence adherence or brand preference.
Visualize operational processes:
Service blueprinting complements JMs by exposing backstage processes and support mechanisms needed to deliver a seamless experience[8].
Overall, journey mapping provides a structured method for understanding the complex and emotional path patients traverse, enabling marketers to design interventions that feel personal and supportive rather than intrusive.
Regulatory Framework: Marketing vs. Care Communications
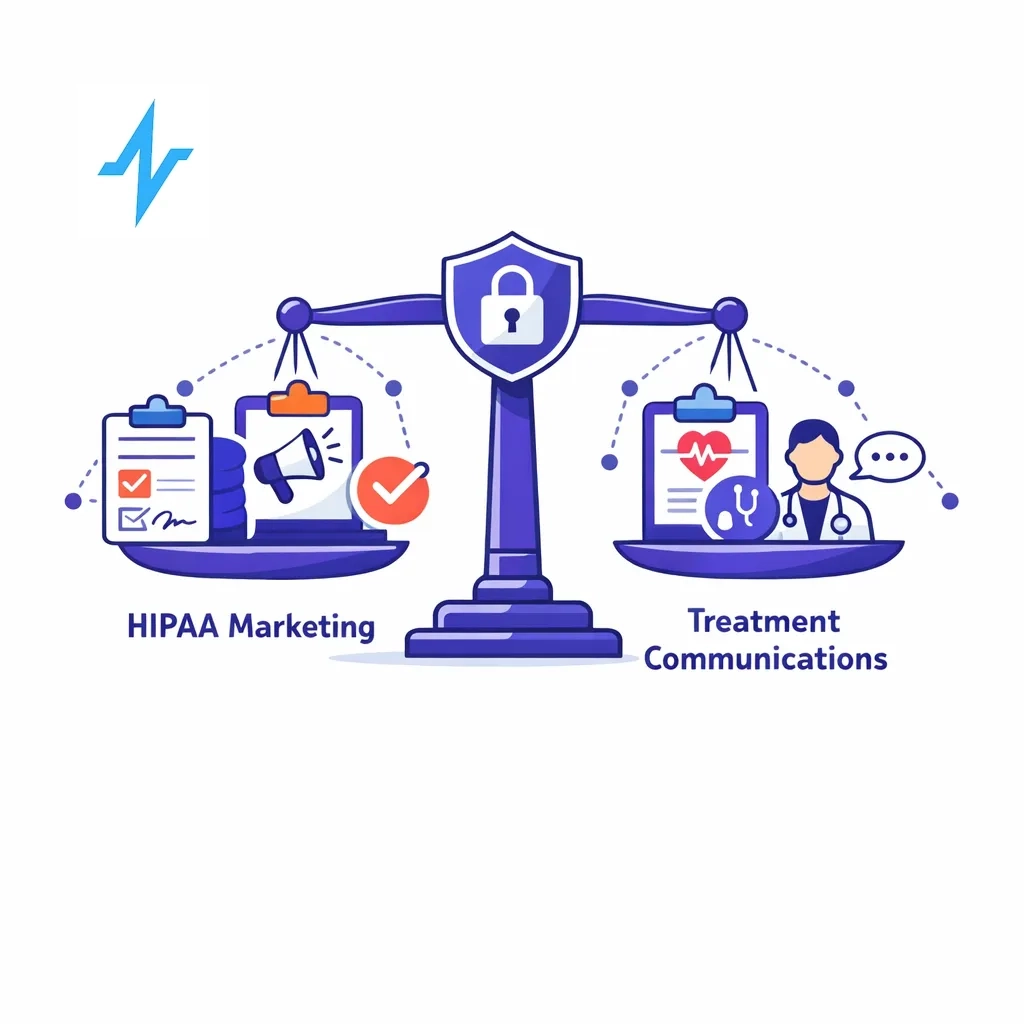
Personalization must respect privacy and legal boundaries. The U.S. Health Insurance Portability and Accountability Act (HIPAA) governs how protected health information (PHI) can be used. The HIPAA Privacy Rule gives individuals control over how their PHI is used for marketing and generally requires written authorization before PHI can be used for marketing communications[9].
HIPAA defines marketing as “a communication about a product or service that encourages recipients…to purchase or use the product or service.”[10]. If a covered entity wishes to sell lists of patients or disclose PHI to a third party for that party’s marketing, it must obtain authorization[11]. Examples of marketing that requires authorization include sending discount coupons for a new drug to patients on a provider’s list[12].
Importantly, the Privacy Rule identifies communications that are not considered marketing and therefore do not require additional authorization. These include communications about the provider’s own health‑related products or services, such as letting patients know about new specialties or equipment[13]; treatment communications, like prescription refill reminders or referrals[14]; and case management or care coordination, such as recommending alternative treatments or settings[15]. For these exceptions to apply, the communications must be part of treatment or care operations and must otherwise be permissible under HIPAA[16].

Pharmaceutical marketers should treat AI‑driven CRM programs as business associate arrangements and ensure agreements limit the use of PHI to permitted purposes[16].
When communications cross into marketing — e.g., promoting a drug outside of prescribed treatment — patient authorization is required.
Complying with HIPAA not only avoids penalties but also builds trust.
Beyond HIPAA, the Trusted Exchange Framework and Common Agreement (TEFCA) aims to create a nationwide network‑of‑networks for health information exchange. Launched by the Office of the National Coordinator for Health IT, TEFCA establishes a universal floor for interoperability, allowing providers, payers, public health agencies, and patients to securely share health information regardless of where it is stored[17]. TEFCA reduces the need for multiple one‑off connections and includes provisions to strengthen data privacy and security[18]. Pharmaceutical marketers should monitor TEFCA developments because easier, standardized access to health data (with consent) will expand the data available for personalization.
The Promise of AI‑Driven CRM in Healthcare
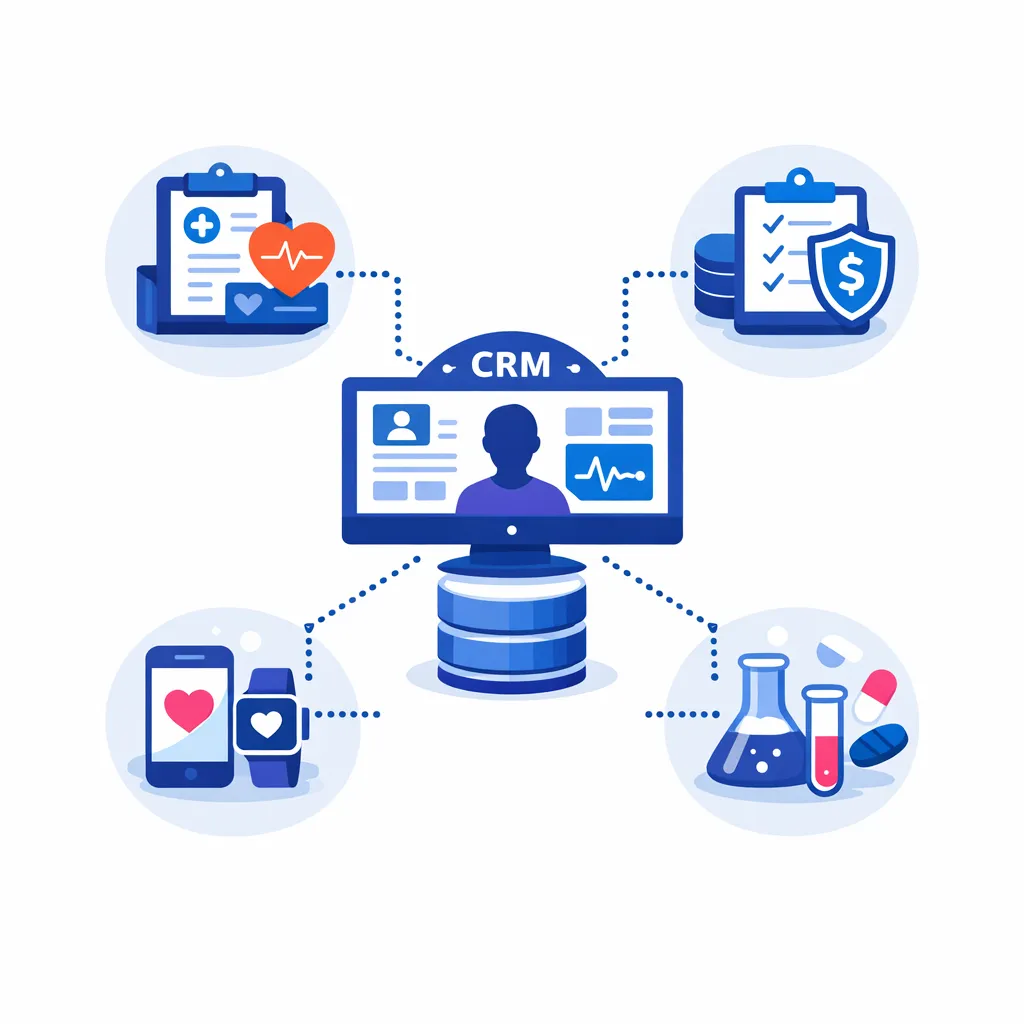
Modern health‑care CRM platforms integrate data from electronic health records (EHRs), claims, mobile apps, and marketing systems to create a unified view of each patient. According to Salesforce’s healthcare CRM guide (an industry commentary), such systems serve as a central hub for patient data, enabling authorized professionals to access information and personalized communication tools[19].
Benefits include enhanced patient relationships, effective data management, streamlined communication, improved engagement and satisfaction, and increased efficiency through workflow automation[20].
A HIPAA‑compliant CRM can also coordinate doctor referrals and proactively tailor messages to patients’ needs[21].

AI supercharges this CRM foundation. In McKinsey’s analysis, AI enables organizations to unlock insights from previously inaccessible data and reimagine the consumer experience[22]. Healthcare generates roughly 30 percent of global data, and this data is growing at 36 percent annually[23]. Patients are also willing to share data: 74 percent would grant access to their personal health information to their primary care providers[24]. AI can analyze this data to predict clinical and behavioural risks and tailor wellness programs[25]. McKinsey estimates that AI could deliver net savings of 5–10 percent of healthcare spending, with variation across payers and providers[26].
Practical Applications of AI in CRM
AI‑driven CRM platforms support personalization through several capabilities:
Segmentation and predictive analytics:
Machine‑learning models identify patient segments based on clinical data, demographics, and behaviors.
For example, AI can predict which patients are likely to be non‑adherent to therapy.
A 2025 systematic review on AI‑based adherence tools found that randomized trials reported improvements in medication adherence ranging from 6.7 percent to 32.7 percent compared to controls[27].
Digital interventions using video and voice for real‑time monitoring showed potential for alerting self‑medication errors[28]. AI models can also forecast the next best action — such as sending a reminder or scheduling a follow‑up — based on patterns in similar patients.
Natural‑language processing and conversational AI:
Generative AI can interact with patients via chat or voice, answering questions about treatment and side effects.
In a study cited by McKinsey, evaluators preferred AI‑generated responses to physician answers on online health forums, rating them higher in quality and empathy[29].

While AI cannot replace providers, it can scale personalized communication, triage inquiries, and gather feedback.
Journey orchestration:
AI can trigger messages at the right time and channel.
For example, the system might send educational content after diagnosis, adherence reminders during treatment initiation, and lifestyle tips during maintenance.
Journey orchestration ensures communications align with HIPAA’s treatment and care‑coordination exceptions; marketing messages can be segregated and sent only with authorization.
Real‑time decision support:
By integrating with EHRs and claims, AI‑driven CRM can flag drug interactions, suggest cost‑effective options, or highlight financial assistance programs.
For marketers, decision support helps align messaging with clinical realities and ensures that promotions complement, rather than contradict, providers’ advice.
Continuous learning:
AI models improve over time as more data becomes available.
Patient interactions — whether through digital channels, call centers, or in‑office visits — create feedback loops.
Systems can adapt segmentation and content, reducing irrelevant communications and increasing relevance.
What’s New in 2026
Several developments in recent years make AI‑driven personalization particularly timely for 2026:
Generative AI adoption is accelerating:
McKinsey’s 2024 survey shows that while adoption is still nascent, many health‑care leaders plan to scale generative AI for consumer engagement[1].
Since large language models can generate empathetic responses and synthesize unstructured data, they broaden what’s possible in CRM.

AI tools for adherence are gaining evidence:
The Frontiers review highlights early but promising findings that AI‑enabled tools improve adherence[27].
Expect a growing ecosystem of digital therapeutics and smart devices feeding CRM platforms.


Increased focus on patient voice:
FDA’s patient‑focused drug‑development guidances encourage systematic collection of patient experience data to inform development and regulatory decisions[31].
This emphasis on the patient’s voice aligns with personalized marketing; marketers must ensure that communications reflect real patient needs rather than assumptions.
Common Mistakes and Misconceptions
- Treating CRM as a marketing tool only. A healthcare CRM is not just a marketing database. It is a coordination platform that supports care operations and must comply with HIPAA. Communications about treatment or care operations are allowed without additional authorization[32], but promotional messages encouraging product use require authorization[33]. Marketers often blur this line; working closely with compliance teams is essential.
- Using consumer CRM systems that lack healthcare features. Generic CRM platforms may not support HIPAA compliance, may fail to integrate with EHRs, and may not provide necessary audit trails. Choosing a healthcare‑specific CRM ensures that data is stored and transmitted securely and that the platform supports features like consent management and authorization tracking[21].
- Ignoring patient journey mapping. Personalization without a clear map can lead to overwhelming patients with irrelevant content or misaligned timing. Investing in journey mapping and involving patients in the process[4] prevents missteps and ensures messaging aligns with real needs.
- Over‑reliance on AI without human oversight. AI models can suggest actions, but human review is necessary to ensure recommendations are clinically appropriate and empathetic. The Frontiers review warns that evidence for AI adherence tools is still limited and subject to bias[34]. Combining AI with human expertise mitigates risks.
- Failing to plan for data governance and consent. AI requires large, integrated data sets. Without robust data governance — defining data sources, consent status, sharing agreements, and retention policies — organizations risk violating privacy rules and undermining trust. Leveraging TEFCA will require careful alignment with its privacy and security principles[18].
Building an AI‑Driven CRM Strategy: A Checklist for Pharma Marketers
The following steps provide a structured approach to personalizing patient journeys:
Clarify your objectives. Determine whether the goal is patient education, adherence support, HCP engagement, or brand awareness. Each goal will influence the type of data needed and the regulatory status of communications.
Map the patient journey collaboratively. Use journey mapping and service blueprinting tools to understand stages, emotions, and touchpoints[2].
Involve patients, caregivers, nurses, and medical affairs colleagues to capture diverse perspectives and co‑create solutions[4].

Build a compliant data foundation. Inventory available data sources (EHR, claims, CRM, websites, call centers). Identify which data are considered PHI and require authorization for marketing. Use consent management tools to store and update authorizations. Align data governance with TEFCA and internal IT policies to ensure interoperability and security[17][18].
Select a healthcare‑specific AI‑enabled CRM. Choose platforms designed for HIPAA compliance with features like consent tracking, audit logs, and integration with EHRs and TEFCA networks.
Evaluate vendors on AI capabilities such as predictive analytics, journey orchestration, and conversational AI.
Develop content and workflows. Create content tailored to each journey stage — educational resources, adherence reminders, financial assistance information, and community support. Use AI to recommend the next best action but establish human review to ensure accuracy and empathy. For marketing messages, obtain and document patient authorization.[33]
Train and align teams. Educate marketing, medical affairs, IT, and legal teams on HIPAA rules, TEFCA, and the distinctions between treatment communications and marketing.
Provide training on AI tools and explain how models arrive at recommendations to build trust and accountability.
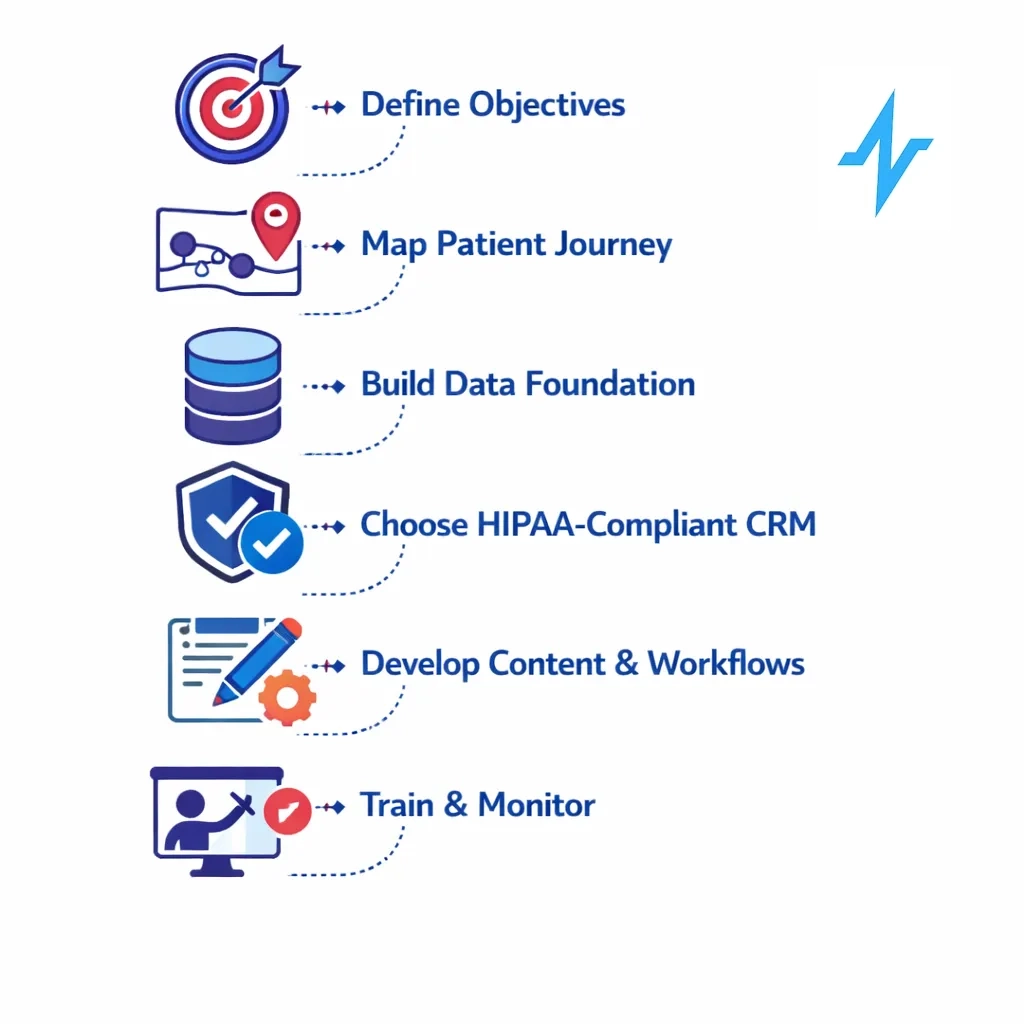
Monitor outcomes and iterate. Use CRM analytics to track engagement, adherence, satisfaction, and business metrics such as conversion rates or prescription refills. AI models can identify segments with low response and suggest adjustments. Regularly revisit patient journey maps to incorporate new insights and adjust strategies.
The Role of Pulse Health
Pulse Health’s AI‑driven healthcare CRM is designed specifically for pharmaceutical marketers seeking to personalize patient journeys. Built on a HIPAA‑compliant architecture, Pulse Health integrates data across EHRs, claims, and marketing platforms, enabling a 360‑degree view of patients.

Pulse leverages machine‑learning models to segment audiences, predict adherence risks, and orchestrate cross‑channel journeys while maintaining strict authorization controls.
And because Pulse Health aligns with TEFCA standards, it can securely connect to Qualified Health Information Networks as they become available, expanding data availability and ensuring interoperability.
By adopting an AI‑driven CRM like Pulse Health, pharma marketers can deliver timely, relevant information that improves patient outcomes, increases satisfaction, and supports brand objectives — all within an ethical and compliant framework.
Personalize More Patient Journeys with Pulse
Personalizing patient journeys is no longer optional for pharmaceutical marketers; it is essential for delivering value in a saturated and regulated market. Journey mapping provides a research‑based framework to understand patient needs and design empathetic interventions[2].
HIPAA, with its clear distinction between marketing and treatment communications[33][32], underscores the importance of compliance and patient consent. TEFCA is creating a nationwide network that will expand data availability and facilitate interoperability[17]. AI‑driven CRM systems harness burgeoning health‑care data to deliver personalized experiences and improve outcomes[22][35]. Early evidence shows that AI tools can boost medication adherence[27], while industry analyses forecast significant efficiency gains[26].
By following a structured strategy — grounded in journey mapping, regulatory compliance, data governance, and AI‑enabled technology — pharmaceutical marketers can transform how they engage patients and HCPs.
The result is a personalized, compliant, and effective experience that benefits patients, providers, and brands alike.

If you’re ready to explore how AI‑driven CRM can transform your patient engagement strategy, consider booking a demo or requesting a consultation with Pulse Health. The journey toward personalized healthcare begins with the first step.

[1] [22] [23] [24] [25] [26] [29] [35] How AI in healthcare can improve consumer experiences | McKinsey
[2] [3] [4] [5] [6] [7] [8] Using Journey Mapping and Service Blueprinting to Design Digital Health Behavior Change Innovations: A Scoping Review – PMC
https://pmc.ncbi.nlm.nih.gov/articles/PMC12061902
[9] [10] [11] [12] [13] [14] [15] [16] [32] [33] Marketing | HHS.gov
https://www.hhs.gov/hipaa/for-professionals/privacy/guidance/marketing/index.html
[17] [18] [30] TEFCA – ASTP – Assistant Secretary for Technology Policy
https://healthit.gov/policy/tefca/
[20] [21] Healthcare CRM Software: A Complete Guide | Salesforce AP
https://www.salesforce.com/ap/healthcare-life-sciences/healthcare-software/healthcare-crm-guide
[27] [28] [34] Frontiers | Artificial intelligence-based tools for patient support to enhance medication adherence: a focused review
https://www.frontiersin.org/journals/digital-health/articles/10.3389/fdgth.2025.1523070/full
[31] FDA Patient-Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient’s Voice in Medical Product Development and Regulatory Decision Making | FDA