Pharma marketers don’t just compete for attention — they compete for trust. In regulated industries, a clever subject line or snappy text is never worth a compliance misstep. This playbook lays out how to design, operate, and scale email and SMS programs that put compliance first without sacrificing performance.
The Regulatory Map (and What it Means in Practice)
United States (high‑level):
- FDA promotion & “fair balance” for Rx products applies to digital, including email and SMS. FDA’s social/character‑limited guidance expects benefit and risk information to appear together, with prominent linkage to fuller risk/PI when space is tight. If you can’t disclose adequate risk in a short message, don’t use that channel for that claim. U.S. Food and Drug Administration
- HIPAA (Privacy & Security Rules) governs PHI. Marketing uses of PHI typically require a patient authorization (with limited exceptions), and all ePHI handling must follow security safeguards. Email/SMS for care is allowed with reasonable safeguards; marketing is different and usually requires written authorization. HHS.gov+2HHS.gov+2
- TCPA governs marketing texts/calls. You need proper consent for marketing texts and must honor opt‑outs (e.g., “STOP”) — revocations must be processed within a reasonable time not to exceed 10 business days. eCFR
- CAN‑SPAM covers commercial email: no deceptive headers, include a physical postal address, and provide a functioning unsubscribe honored within 10 business days. Federal Trade Commission+1
- CTIA carrier rules (industry best practices) shape how A2P traffic is treated: clear calls‑to‑action, documented opt‑in, and standard commands like STOP/HELP that must work across carriers. CTIA API
- 10DLC registration (A2P): U.S. mobile carriers require brand/campaign registration via The Campaign Registry (TCR) to send application‑to‑person texts over local numbers. Unregistered traffic is throttled/blocked. Campaign Registry+1
EU/UK highlights (if you message there):
- GDPR Article 9 treats health data as “special category” data — processing generally needs explicit consent (or another narrow legal basis). GDPR
- ePrivacy/PECR requires consent for direct marketing by email/SMS, along with easy, free withdrawal (think: one‑tap unsubscribe). The UK ICO’s guidance explains consent standards and the “soft opt‑in” nuances. EUR-Lex+2ICO+2
Bottom line: map every program to the strictest applicable regime for your audience and content type. When in doubt, collect explicit, recorded consent and make opt‑outs effortless.
Content First: Designing Messages that Pass MLR and Delight Recipients
1) Promotional vs. non‑promotional flows
- Care/operational messages (e.g., appointment reminders, adherence nudges) can often proceed with patient preferences and standard HIPAA safeguards.
- Marketing (e.g., patient support program offers, product announcements) typically requires written authorization when PHI is involved. Document the difference in your SOPs and templates. HHS.gov

2) “Fair balance” for Rx in short formats
If you cite benefits or make product claims, include risk information in the same message and a prominent path to the full PI/ISI (e.g., a branded short URL to a risk‑only landing page). If there isn’t room to do both clearly, don’t send it by SMS — use email with a compliant layout or drive to a compliant page first. U.S. Food and Drug Administration
3) Standard compliance blocks that travel with the message
Embed (and lock) components that MLR approves once and reuse everywhere:
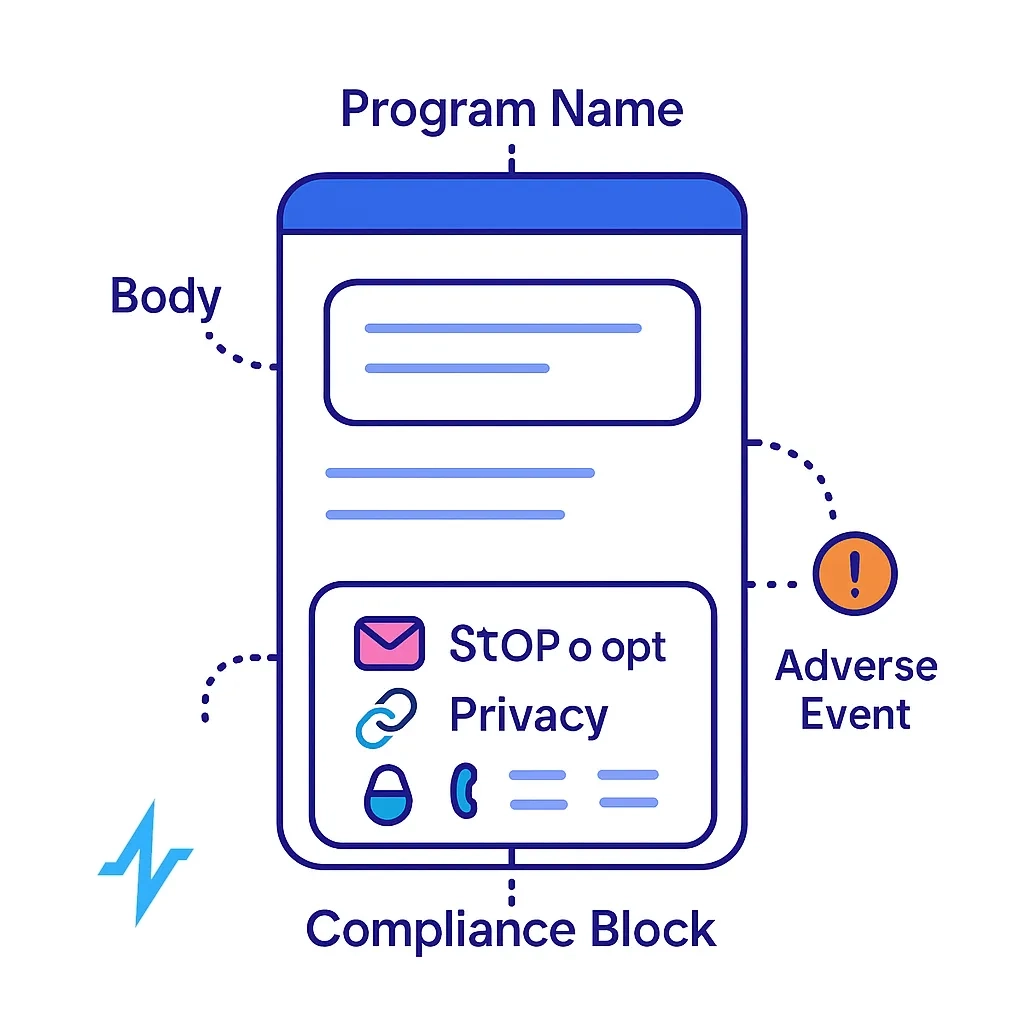
- Program name + sender identity
- “STOP to opt out, HELP for help,” with functional handling
- Link to privacy policy and PI/ISI (where applicable)
- Contact details / postal address (email); support contact for SMS
- Adverse Event (AE) intake notice (see PV section below)
These elements align with CTIA, CAN‑SPAM, and FDA expectations for transparency. CTIA API+2Federal Trade Commission+2
Consent, Opt‑out, and Preference Management
- Capture: Use clear, conspicuous calls‑to‑action that name the brand/sender, specify message types/frequency, and record evidence (timestamp, page/context, IP, disclosure text). CTIA spells out what to store. CTIA API
- Honor revocation everywhere: Accept “STOP,” “UNSUBSCRIBE,” “QUIT,” etc., and turn them off across all linked campaigns. Under TCPA, revocations (by any reasonable means) must be honored quickly — within 10 business days at most. eCFR
- Evolving TCPA landscape: The FCC in late 2023 adopted rules targeting the “lead generator loophole” (pushing toward seller‑specific consent), but parts were vacated by the Eleventh Circuit in 2025, and the FCC subsequently aligned the rule text. Best practice for healthcare remains clear, brand‑specific consent tied to the topic. Coordinate with counsel on your forms and brokered leads. Federal Register+2Consumer Financial Services Law Monitor+2

Pharmacovigilance (PV): Don’t Miss Safety Signals
Any reply, email, or inbound that suggests an adverse event must route to PV promptly and be logged.
For marketed drugs/biologics in the U.S., serious & unexpected AEs known to the applicant must be reported to FDA as soon as possible and no later than 15 calendar days from initial receipt (biologics follow a parallel rule).
Build your capture/escalation workflows accordingly. eCFR+1
Security, Privacy, and Vendor Governance
- HIPAA Security Rule: apply administrative, physical, and technical safeguards to protect ePHI (think MFA, access control, audit logs, encryption in transit — and at rest where appropriate). HHS.gov
- Encryption: Under current rules, certain encryption controls are “addressable” (decide via risk analysis and implement or document an equivalent measure), but HHS has proposed making encryption and MFA explicit requirements in a 2025 Security Rule update. Track the NPRM and plan for uplift. eCFR+1
- Business Associate Agreements (BAAs): if a vendor touches ePHI (ESP, SMS gateway, link shortener, analytics), you likely need a BAA with the required clauses at 45 CFR 164.504(e). Don’t send PHI through a vendor without one. HHS.gov+1
10DLC, Sender Identity, and Deliverability Hygiene
- Register A2P traffic (brand + campaign) via TCR and ensure your use case (e.g., healthcare notifications, marketing) matches your traffic profile. This is now a practical prerequisite for throughput and deliverability in the U.S. Campaign Registry
- Use the right rails: short codes for very high‑volume or sensitive patient programs; branded 10DLC for scaled conversational flows; dedicated toll‑free where appropriate. CTIA best practices and carrier policies govern blocking/suspension for unwanted traffic. CTIA API
What “Good” Looks Like (Safe Patterns)
A. Patient service (non‑marketing) SMS
[Pulse Health Cardiology]Appt reminder for 11/14 at 10:30am with Dr. Singh. Reply C to confirm or R to reschedule.
Msg&data rates may apply. STOP to opt out, HELP for help.
- Purpose‑limited, no promotion; clear controls (STOP/HELP); minimal PHI in the message.
B. Promotional email for an Rx (consumer audience)
- Subject/body includes product name + indication, a concise most‑serious‑risks “ISI blurb” near benefits, persistent footer with full ISI/PI links, privacy policy, postal address, and one‑click unsubscribe. Avoid claims that can’t be fairly balanced in‑line. U.S. Food and Drug Administration+1
C. HCP email
- Clinical claim with citation, appropriate risk context, link to full PI, and an HCP‑only statement. Avoid off‑label; ensure the claim aligns to labeling and MLR‑approved copy. U.S. Food and Drug Administration
Pulse Health’s Compliance‑First Build Checklist
Strategy & governance

- Define message classes (care vs. marketing) with routing rules and MLR workflows.
- Maintain jurisdictional rules of the road (U.S., EU, UK) in a living playbook. ICO+1
Consent & identity
- CTIA‑compliant calls‑to‑action; store consent receipts; support STOP/HELP; global suppression lists; TCR/10DLC registration complete. CTIA API+1

Content controls
- Modular templates with locked compliance blocks, ISI/PI links, and channel‑appropriate risk/benefit balance logic. U.S. Food and Drug Administration
Security & privacy
- Role‑based access, audit trails, TLS, at‑rest encryption (per risk analysis), DLP link wrapping, and BAAs in place for all PHI‑touching vendors. Track HHS’s proposed Security Rule changes (encryption/MFA). eCFR+2Federal Register+2

PV & monitoring

- AE keyword detection on replies and inboxes; automated PV tickets with required data elements; 15‑day expedited reporting timeline awareness; quarterly quality checks. eCFR
Operations
- Data retention & minimization policies; suppression sync across email/SMS; deliverability monitoring; complaint handling SOPs; periodic audits against CTIA/CAN‑SPAM/TCPA. CTIA API+2Federal Trade Commission+2
Common Pitfalls to Avoid
- Cramming claims into SMS without room for risk disclosure — use email or a landing page step. U.S. Food and Drug Administration
- Treating care messages like marketing (or vice versa) — the HIPAA authorization bar differs. HHS.gov
- Unregistered A2P traffic — expect throttling/blocks and carrier scrutiny. Campaign Registry
- Unclear or buried opt‑outs — carriers and regulators expect unambiguous, functional controls (STOP/HELP; one‑click unsub). CTIA API
The Pulse Health Take
Compliance‑first is a growth strategy. When every touch respects consent, presents risk/benefit honestly, and routes safety signals correctly, you earn durable engagement — and fewer headaches for brand, legal, and PV.
If you’d like, we can adapt this playbook into your internal SOPs, consent language, and MLR‑approved templates — for consumer and HCP programs — tailored to your jurisdictions and product portfolio.
Legal note: This article is informational and not legal advice. Regulations evolve (for example, recent TCPA and HIPAA Security Rule developments). Always confirm requirements with your legal and compliance teams. Consumer Financial Services Law Monitor+1
Key sources: FDA social/character‑limited guidance; HHS HIPAA privacy/security materials; FTC CAN‑SPAM; eCFR TCPA rules; CTIA Messaging Principles; GDPR/ePrivacy/ICO guidance; The Campaign Registry.