Empowering Pharma and Healthcare Organizations with Secure Marketing Solutions
Effective communication in the life sciences sector is more than a business requirement — it’s a critical regulatory necessity. Pharmaceutical organizations, healthcare providers, and life sciences firms must navigate a complex landscape of compliance mandates to securely handle sensitive patient and healthcare professional (HCP) data.
Pulse Health is uniquely designed to address these challenges head-on, combining secure communications, comprehensive compliance measures, and seamless CRM integrations.
The Regulatory Landscape in Life Sciences
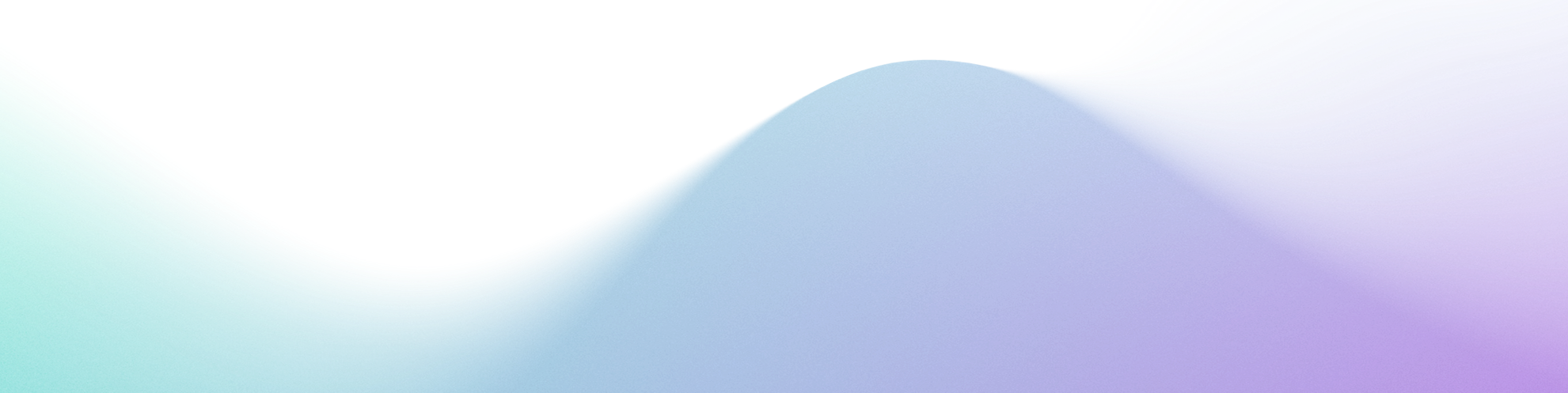
Life sciences communications are strictly regulated, with frameworks including HIPAA, FDA 21 CFR Part 11, and GDPR governing data privacy and protection.
HIPAA ensures patient data privacy, requiring healthcare entities to implement safeguards to protect medical records and other personal health information.
FDA 21 CFR Part 11 governs electronic records and signatures, requiring secure and traceable data handling practices.
GDPR, relevant for global operations, mandates stringent privacy controls and transparency in data usage.
These regulations mandate secure call recording, thorough documentation of communications, robust data handling, and stringent privacy standards to protect patient and HCP information.
HIPAA Compliance Requirements
Protects patient health information in the U.S.; mandates administrative, physical and technical safeguards (e.g. encryption, access controls, audit logs) for any recorded or stored PHI.
Key HIPAA Requirements
- Technical safeguards: encryption of PHI in transit and at rest; unique user IDs; audit trails for all access.
- Administrative safeguards: policies, workforce training, risk assessments.
- Physical safeguards: facility access controls, device/media controls for call-recording storage.

FDA 21 CFR Part 11 Compliance Requirements
Governs FDA-regulated electronic records and signatures; requires system validation, immutable timestamped audit trails, and secure e-signature linking for any data used in clinical or manufacturing processes.
Key FDA 21 CFR Part 11 Requirements
- Record retention: maintain electronic records in their original format for specified periods.
- System validation: ensure software and hardware perform as intended.
- Audit trails: immutable, timestamped logs of record creation, modification, and deletion.
- Electronic signatures: unique and linked to their records, with controls to prevent forgery.

GDPR Compliance Requirements
Covers personal data of EU residents; demands a lawful basis for processing (e.g. consent), data-subject rights (access, erasure, portability) and breach notification within 72 hours—so call recordings require clear consent, transparency, and the ability to fulfill erasure requests.
Key GDPR Requirements
- Breach notification: report serious breaches to authorities within 72 hours and to affected individuals without undue delay.
- Lawful basis for processing: consent, contract, legal obligation, vital interests, public task, or legitimate interests.
- Data-subject rights: access, rectification, erasure (“right to be forgotten”), portability, restriction, objection.
- Privacy by design & default: embed data-protection measures into systems from the outset.

Common Security & Compliance Challenges in Healthcare Marketing
Companies frequently encounter multiple challenges when striving to maintain compliant communications:
- Unencrypted Channels: Traditional communication methods can expose sensitive data due to lack of encryption. Calls and messages sent without encryption can be easily intercepted, potentially leading to unauthorized disclosures.
- Incomplete Audit Trails: Missing metadata or incomplete documentation can lead to non-compliance issues during audits. Without comprehensive audit trails, organizations struggle to verify who accessed specific records and when, complicating compliance reporting.
- Fragmented Systems: Disconnected platforms often create gaps in documentation, increasing compliance risk.
Without integrated systems, vital information can slip through the cracks, making comprehensive compliance oversight challenging. - User Access Management: Misconfigured permissions and inadequate role definitions may lead to unauthorized data exposure.
Organizations must carefully manage access to sensitive data to mitigate internal threats.
Pulse Health’s Compliance-First Architecture
Pulse Health is engineered with compliance at its core, addressing key regulatory requirements seamlessly:
End-to-End Encryption:
Pulse Health uses Transport Layer Security (TLS) for data in transit and AES-256 encryption for data at rest, ensuring sensitive patient and HCP interactions remain secure from unauthorized access or interception.

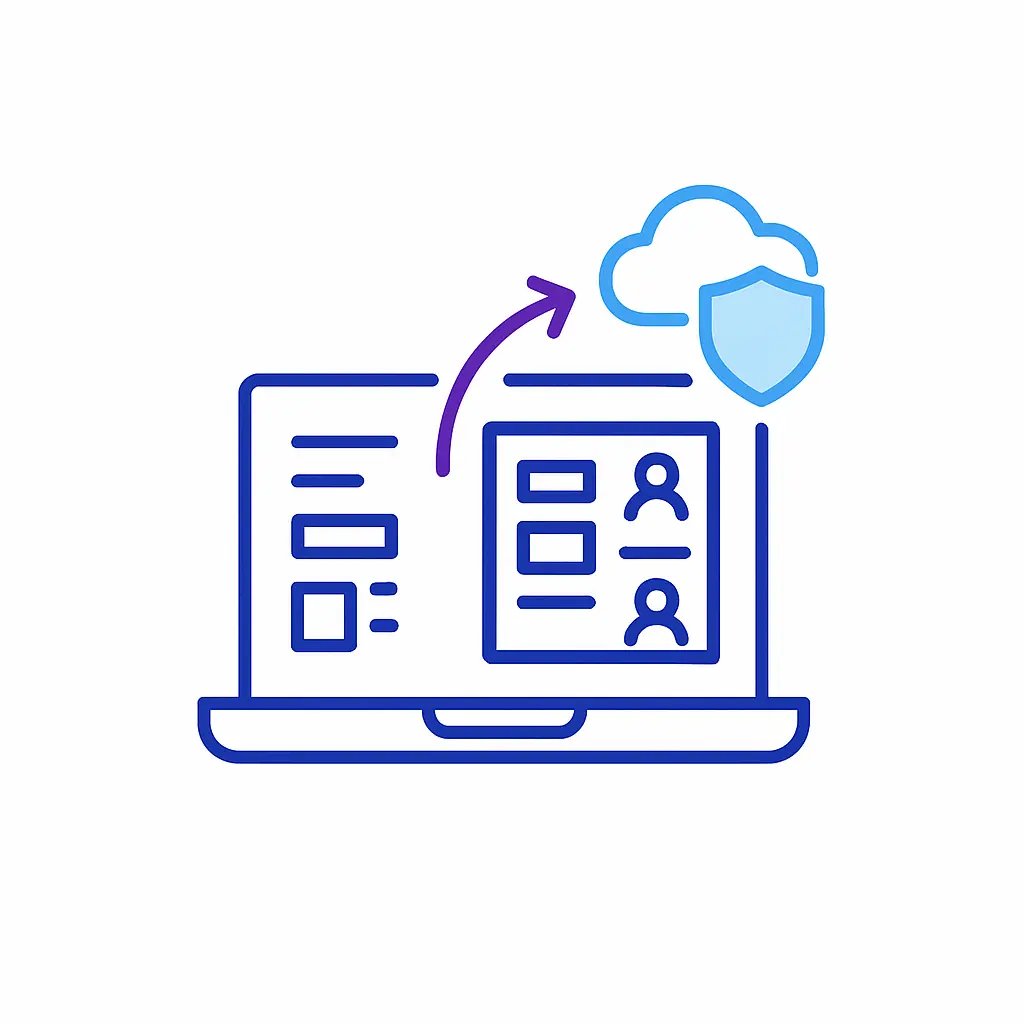
Automated Audit Logging:
Every communication is automatically logged with comprehensive metadata, including timestamps, user actions, and CRM synchronization events, providing thorough documentation required for regulatory audits.
These detailed logs significantly simplify compliance reporting and audit preparation.
Role-Based Access Controls:
Pulse Health offers granular permissions that clearly define roles for administrators, managers, and customer-facing agents, significantly reducing insider risk and enhancing security.
This structured approach ensures only authorized personnel have access to sensitive data.

Consent & Disclosure Management:
Built-in features prompt users to obtain explicit consent for recorded interactions, supporting compliance with regulations such as HIPAA and GDPR.
These prompts help maintain transparency and trust with patients and HCPs, protecting both parties and the organization itself.
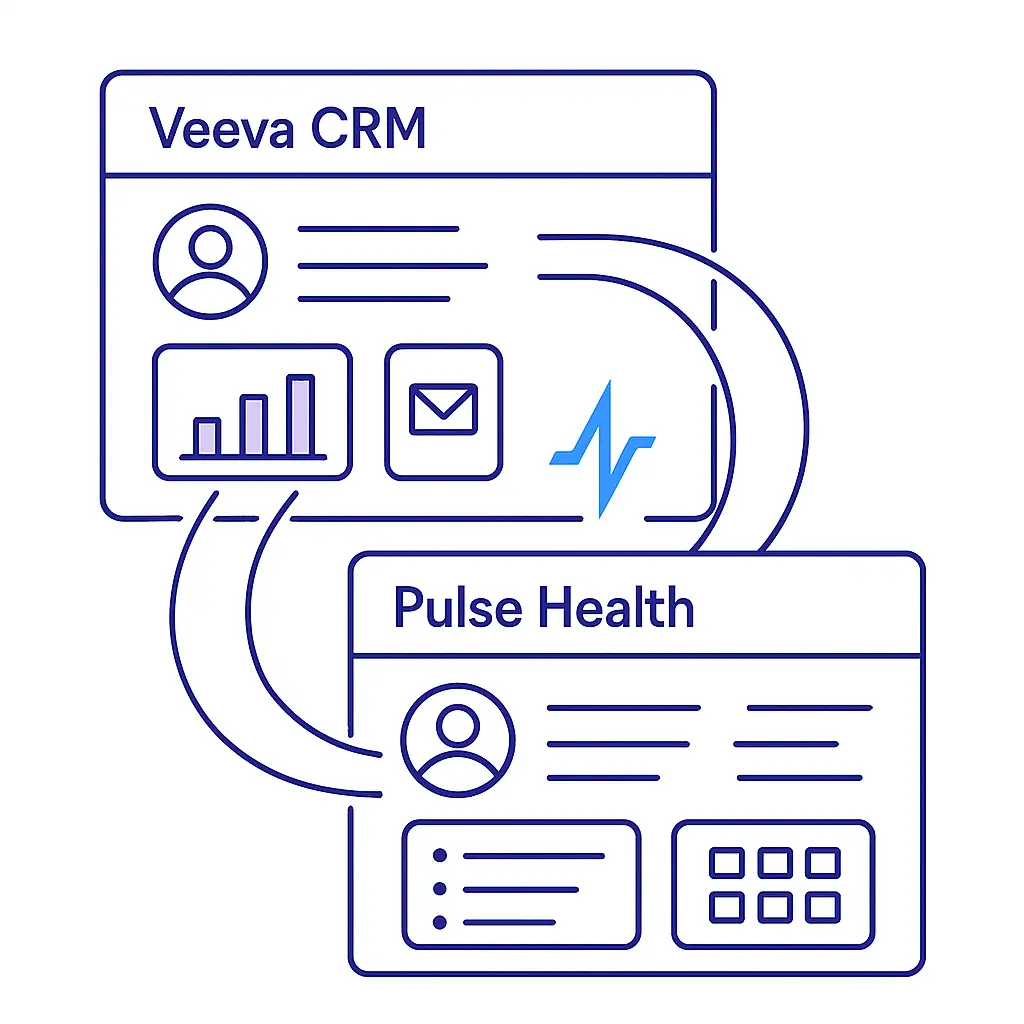
Secure Veeva CRM Integration:
Pulse Health ensures 21 CFR Part 11 compliance by securely synchronizing all interactions and associated data directly with Veeva CRM, including audit history and digital signatures.
This integration ensures seamless compliance reporting and streamlines record-keeping.
Step-by-Step Implementation Guide
Implementing Pulse Health’s compliance framework involves a straightforward but structured process:
- Onboarding & Configuration:
Begin by defining user roles, configuring encryption settings, and integrating the platform seamlessly with your existing Veeva CRM environment. Pulse Health provides guided onboarding, ensuring each step aligns with your specific compliance needs and business processes.
- Staff Training & SOP Alignment:
Align internal Standard Operating Procedures (SOPs) with Pulse Health’s intuitive workflows, providing staff with training tailored to your compliance and security needs.
Regular training sessions ensure your team remains aware of best practices and updated on regulatory changes.
- Testing & Validation:
Conduct pilot interactions, validate audit logs, and complete rigorous quality assurance reviews against regulatory requirements to ensure comprehensive compliance readiness. This proactive approach identifies and addresses potential compliance gaps before they become issues.
Ongoing Governance & Best Practices
Maintaining compliance is an ongoing effort, and Pulse Health supports best practices such as:
- Regular Compliance Audits: Regular audits identify potential compliance gaps, ensuring continuous adherence to regulations.
- Clearly Defined Data Retention Policies: Pulse Health enables clear and enforceable data retention policies, ensuring compliance with regulatory storage requirements.
- Incident Response Planning and Training: Pulse Health supports the development of robust incident response plans, including regular training to prepare teams for swift and effective responses to potential breaches.
- Regular Reviews of User Access and Permissions: Regularly reviewing access controls helps prevent unauthorized access, keeping sensitive information protected and ensuring adherence to compliance standards.
Real-World Use Case
Consider a mid-sized pharmaceutical company facing frequent compliance challenges. Implementing Pulse Health allowed the company to:

- Reduce audit preparation time by 50%, thanks to automated and comprehensive audit logging.
- Prevent a potential HIPAA violation by leveraging built-in consent management tools, significantly reducing legal and reputational risks.
- Successfully demonstrate full 21 CFR Part 11 compliance during an FDA audit due to integrated CRM synchronization and secure data handling, avoiding costly penalties and delays.
Learn how Pulse Health can help you stay in compliance with HIPAA, FDA 21 CFR Part 11, and GDPR regulations by scheduling a demo with our team today.
How Pulse Health Helps You Stay Compliant with Healthcare Regulations
Pulse Health bridges the gap between secure communication and regulatory compliance, empowering life sciences organizations with robust, secure, and fully compliant communication workflows.
By adopting Pulse Health, organizations can proactively address compliance challenges, minimize risk, and confidently navigate complex regulatory landscapes.
If your organization seeks to streamline compliance, secure sensitive data, and enhance regulatory readiness, consider scheduling a personalized demonstration. Or keep reading through our blog to explore further resources and learn how Pulse Health can transform your communication security and compliance practices.