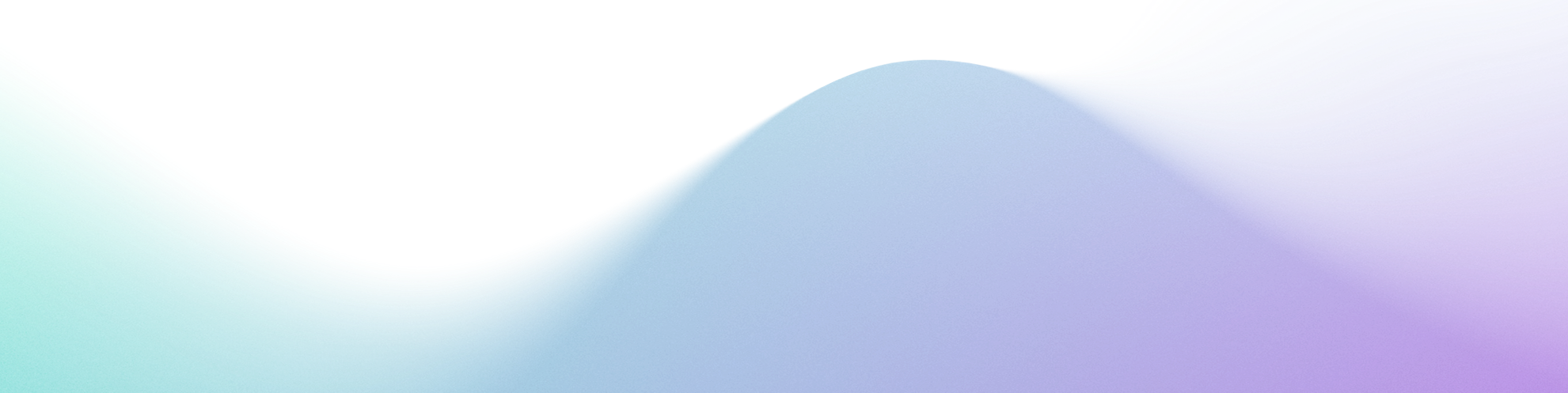
Stop Wasting Budget: How Data Quality Drives ROI in Pharma
Budgets are tight and evidence demands are higher. If your data layer is messy — IDs don’t match, consent is unclear, offline outcomes aren’t connected — your pharma marketing ROI suffers. The fix isn’t “more channels.” It’s better data: collected with consent, stitched across touchpoints, governed by design, and activated quickly.
In this playbook, you’ll get a practical blueprint to unify HCP and patient signals, standardize tracking, and direct spend toward measurable impact — without compromising privacy or FDA/HIPAA requirements.
We’ll also outline a 90-day plan you can start now.
How Pulse Fits In: Pulse Health provides a unified, compliance-first data foundation with identity stitching, consent tracking, pre-built integrations, and a next-best-action engine that turns data into timely HCP and patient interventions.
Why ROI Lags in Pharma (Root Causes)
Even great creative struggles on a weak data layer:

- Silos across media, CRM, field, hub/POC, and patient services
- Identity gaps (incomplete NPI/HCO mapping, brittle device IDs, inconsistent hashed emails)
- Attribution blind spots for offline outcomes (EHR, hub milestones, access barriers)
- Governance drag: unclear ownership of data quality; slow, non-auditable MLR packages
Transition: Real ROI starts by defining what “return” actually means for your brand stage, then collecting the minimum viable data to measure and optimize it.
How Pulse Fits In: Pulse stitches media + CRM + hub events, maintains a consent ledger, and exposes audit-ready history so brand, field, and compliance move faster together.
Defining ROI in Pharma — What Actually “Returns”?
Tie ROI to lifecycle and outcomes you can prove:
- Pre-launch & launch: qualified HCP engagements, reach/quality, early TRx/NRx lift
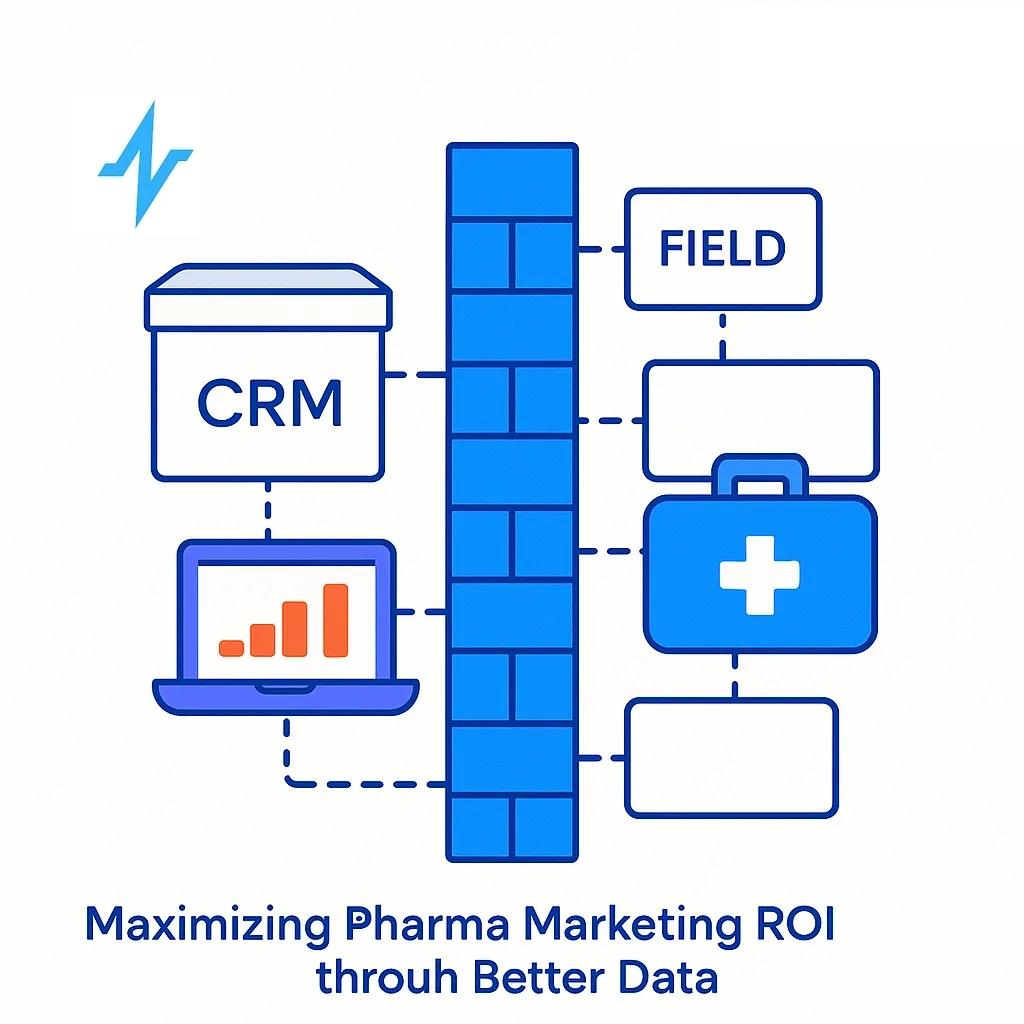
- Growth: access pull-through, rep efficiency, high-value account coverage
- Patient: starts, time-to-therapy, adherence/persistence
Translate into financial lenses: CLV by therapy, LTV:CAC, cost per qualified HCP/patient, and incremental lift validated via experiments.
How Pulse Fits In: KPI templates and dashboards that ladder from activities → quality signals → outcomes (TRx/starts) → dollars.
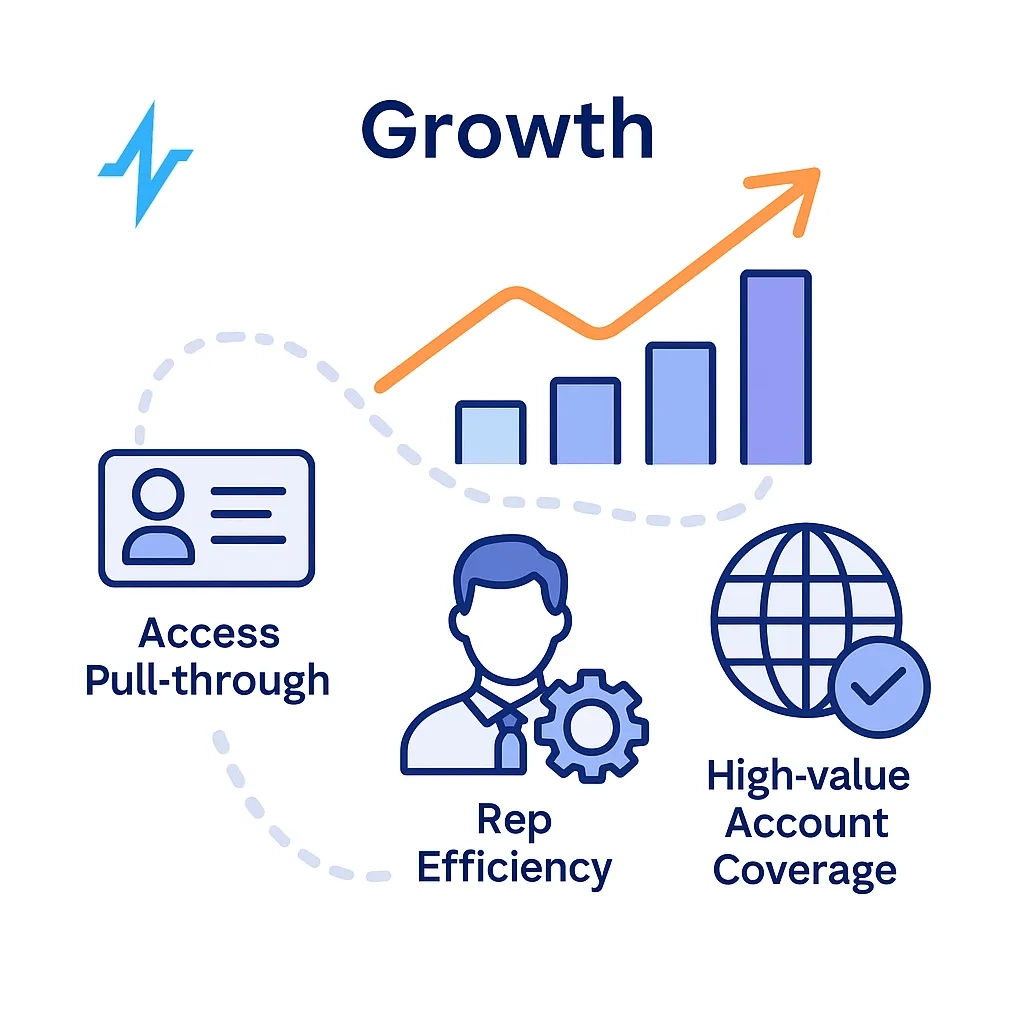
The “Better Data” Pillars (Build These First)
- First-party & consented data: verified identifiers, channel preferences; patient services data under proper legal bases (e.g., BAAs). See HHS sample BAA provisions and tracking-tech guidance for regulated entities. (HHS BAA sample; HHS tracking tech). (HHS.gov)
- Quality & completeness: standardization, deduping, missing-data playbooks, and QA gates at ingestion
- Identity resolution: accurate NPI/HCO mapping (NPI info: CMS NPPES/NPI Registry) with privacy-safe keys. (NPI Registry)
- Interoperability: integrate CDP/CRM/DWH with platforms like Veeva CRM (field/content), Doceree (HCP programmatic), and OptimizeRx (EHR/POC messaging). (Veeva, Doceree, OptimizeRx)
- Governance & compliance: align to HIPAA Privacy/Security Rules and 21 CFR Part 11 (audit trails, e-signatures, trustworthy e-records). (HIPAA Security; eCFR Part 11). (HHS.gov)
How Pulse Fits In: Policy-as-code validation at ingestion, role-based access, retention controls, and pre-built connectors limit risk while speeding activation.
Minimum Viable Data (Collect What You’ll Use)
HCP (non-PHI): NPI, specialty, affiliation, territory, channel preferences, recent engagement, verified contact routes, content interests (map to a consistent taxonomy).
Field: structured call outcomes, sample/rep activity tied to subsequent TRx/NRx where permissible.
Media & Web: clean UTM conventions and a shared content taxonomy (e.g., IAB Content Taxonomy). (GA4 UTM builder; IAB taxonomy). (Google Help)
Patient Services: consent states, enrollment steps, coverage/PA status, start and adherence milestones — tracked with minimum-necessary or de-identified data.
How Pulse Fits In: An opinionated schema + enrichment to fill gaps without over-collecting PHI.
From Better Data to Higher ROI: High-Impact Use Cases
- Omnichannel sequencing / Next-Best-Action (NBA): choose the next content + channel per HCP based on behavior, access, and formulary context
- Audience refinement: privacy-safe look-alikes built from qualified HCP/patient cohorts
- Rep targeting & territories: dynamic call lists with alerts (coverage changes, recent hub triggers)
- Content optimization: claims-approved modular content tagged by indication/claim; learn from MLR outcomes
- Patient support optimization: remove bottlenecks (benefits verification, PA, copay) and nudge to accelerate starts; IQVIA has reported time-to-therapy reductions when PSP processes are streamlined. (IQVIA case/white papers). (IQVIA)
How Pulse Fits In: Real-time event ingestion triggers rules/ML for actions — and suppression to avoid waste.
Attribution Pharma Can Trust (MMM + MTA + Experiments)
Use a layered strategy:
- Experiments (RCTs, holdouts/geo-tests) for causal lift
- MTA for addressable digital paths
- MMM for long-horizon budget mix and offline channels
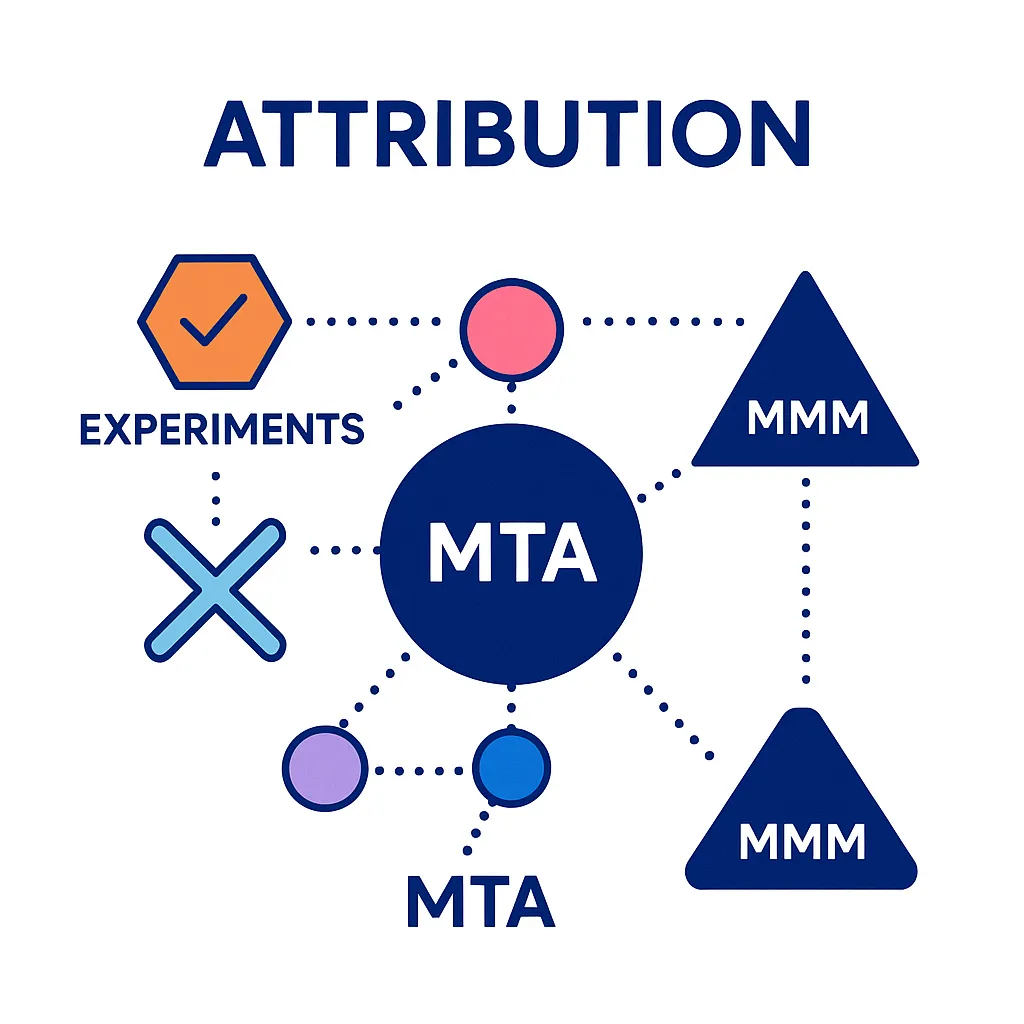
For common definitions and trade-offs, see the IAB guide; for MMM, Meta’s open-source Robyn is a widely used option. (IAB MMM vs MTA; Robyn docs). (IAB)
How Pulse Fits In: Test templates, clean-room connectors, and KPI ladders that trace activity → outcomes → dollars.
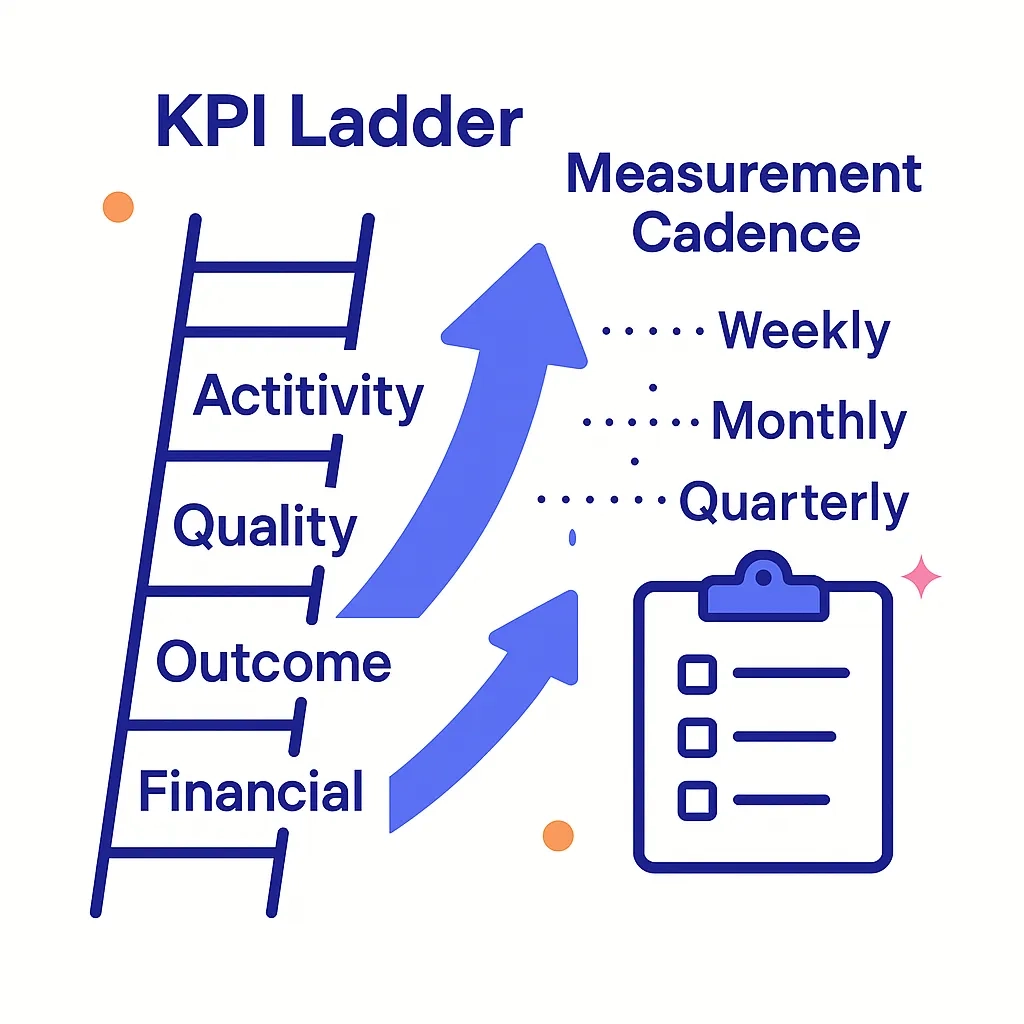
One-Page ROI & Measurement Cadence
North Star: incremental qualified HCP engagements → incremental TRx/starts
OEC (Overall Evaluation Criterion): single score blending efficiency (cost per qualified outcome) + impact (incremental lift)
Cadence: weekly ops dashboard, monthly causal readout, quarterly mix refresh
KPI Ladder (example)
| Layer | KPI Examples | Decision It Informs |
| Activity | Emails delivered, rep calls logged, POC impressions | Coverage & pacing |
| Quality | HCP reach quality, content depth, verified HCP interactions | Sequencing & content |
| Outcome | TRx/NRx lift, starts, time-to-therapy | Budget allocation |
| Financial | LTV:CAC, cost per incremental start | Scale vs. cut |
Experimentation Playbook (Turn Insight into Dollars)

- Prioritize by impact/effort and seasonality (e.g., access cycles); start with message + channel timing and hub/POC orchestration
- Pre-register KPIs; ensure control/exposed parity and adequate power
- Test types: claims-approved content variants, rep-assist scripts for PA hurdles, hub outreach cadence, POC trigger frequencies
How Pulse Fits In: Library of experiment designs, auto-generated tracking sheets, and governance artifacts to keep MLR confident.
Compliance by Design (Non-Negotiables)
- HIPAA: data minimization, role-based access, audit trails; treat tracking vendors carefully when PHI could be touched. (HHS Privacy/Security summaries; HHS tracking tech). (HHS.gov)
- 21 CFR Part 11: validated systems, audit trails, and unique e-signatures for regulated processes. (eCFR Part 11). (eCFR)
- Privacy-enhancing tech: consider data clean rooms for privacy-safe joins and differential privacy for safe aggregate reporting. (IAB DCR guidance; NIST SP 800-226). (IAB Tech Lab)

How Pulse Fits In: Enforces consent provenance, role-based permissions, retention controls, and audit-ready exports for HIPAA and Part 11 reviews.
Common Pitfalls & Fixes
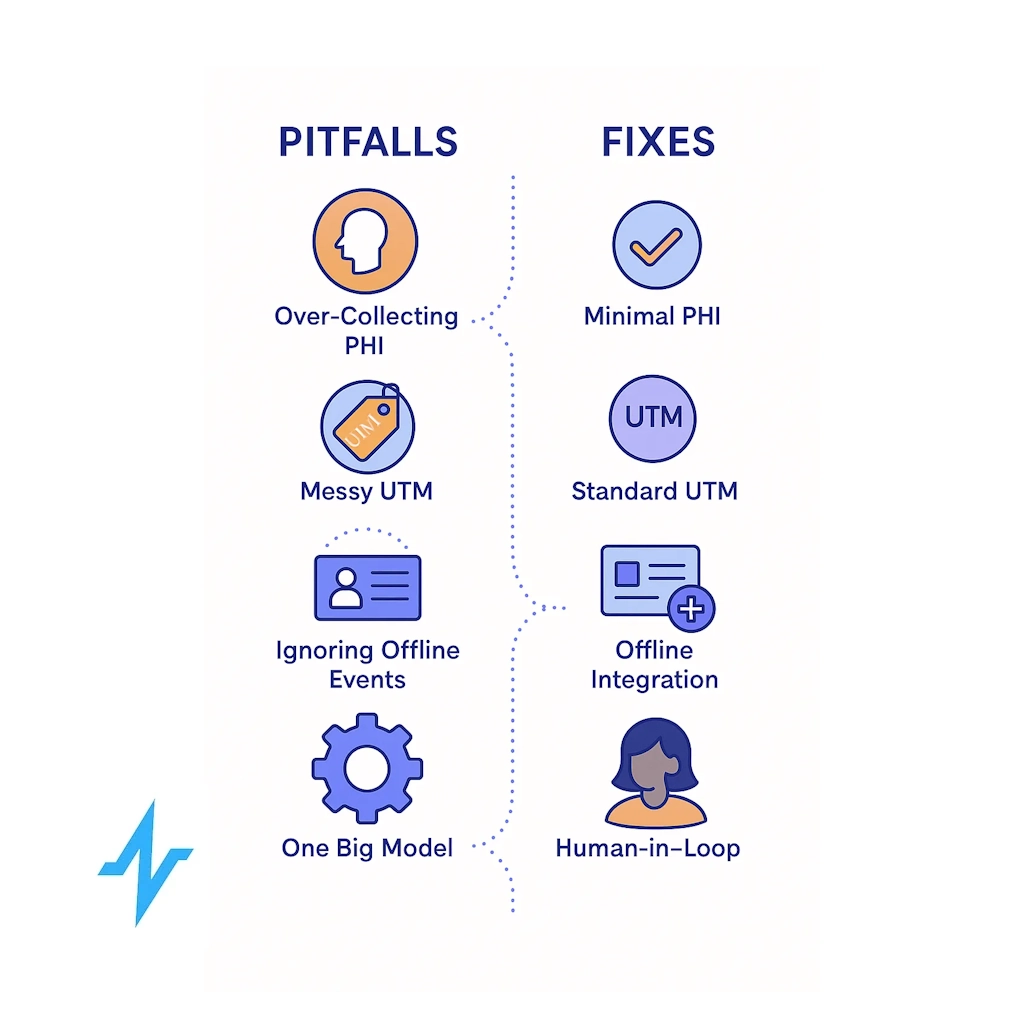
- Over-collecting PHI “just in case.”
- Messy UTM/tagging, breaking channel and content attribution
- Ignoring offline events (hub milestones, payer decisions)
- “One big model” syndrome with no human-in-the-loop
Fixes: purpose-bound schemas, documented UTM standards, SLAs for offline conversion imports, modular ML with clear review cycles. (UTM best practices: GA4 URL builder; content taxonomy: IAB). (Google Help)
How Pulse Fits In: Data quality monitors, drift alerts, and a governance panel shared with compliance/MLR.
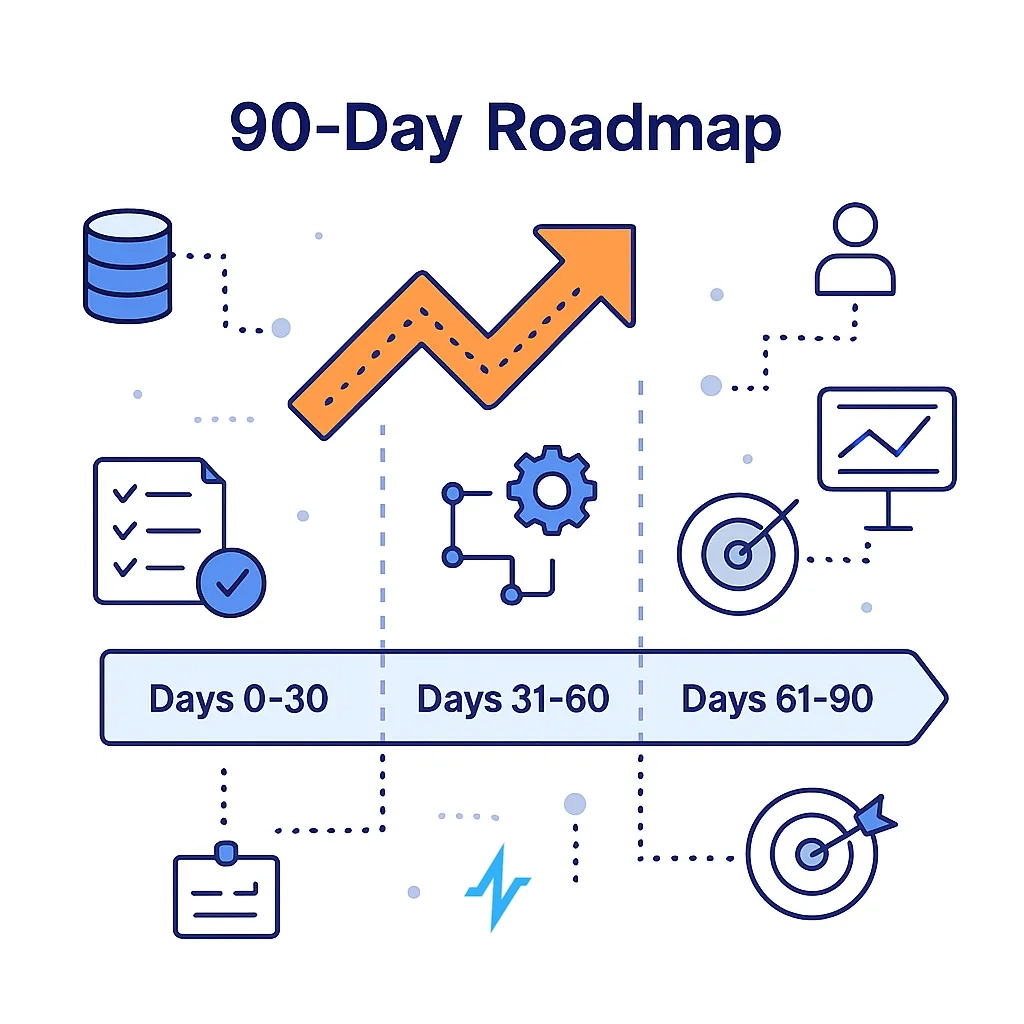
90-Day Roadmap to ROI Uplift
Days 0–30
- Data/consent audit; implement UTM + content taxonomy
- Connect priority systems (CRM, POC, HCP programmatic) and define 3–5 North-Star KPIs
Days 31–60
- Complete identity stitching (NPI/HCO + privacy-safe keys)
- Launch first NBA journeys + 1–2 holdout experiments for causal reads (MMM/MTA plan drafted)
Days 61–90
- Extend to field triggers; import offline conversions (hub events)
- Reallocate budget to measured lift; scale winners, suppress waste
How Pulse Fits In: Onboarding sprints, solution engineers for brand + field, and weekly measurement reviews with actionable recommendations.
Get Started with Pulse Health Today
If you’re ready to translate better data into measurable pharma marketing ROI, Pulse Health brings the essential pieces together: compliant data collection, identity resolution, real-time activation, and a measurement stack your brand, field, and compliance teams can trust.
- Unify your data: Pre-built integrations across CRM/CDP, Veeva, HCP programmatic, and point-of-care
- Act with confidence: Next-best-action journeys that respect consent, claims, and access realities
- Prove impact: Experiments + MTA + MMM, all tied to TRx/NRx, starts, and time-to-therapy

Get Started with Pulse Health Today — and turn every interaction into a step toward faster therapy starts and smarter spend.
Frequently Asked Questions
Start with consented first-party HCP identifiers (NPI/HCO), channel preferences, high-signal engagement events, standardized UTM/taxonomy, and patient-services milestones (minimum necessary). (NPI Registry; IAB taxonomy). (NPI Registry)
HIPAA applies when PHI is involved; treat tracking vendors carefully, maintain BAAs where required, and follow HHS’ tracking-tech guidance for regulated entities. (HHS tracking tech). (HHS.gov)
It governs trustworthy electronic records/signatures for FDA-regulated processes — relevant when marketing systems intersect with regulated workflows, e.g., approvals. (eCFR Part 11). (eCFR)
Yes: use experiments for causality, MTA for granular digital paths, and MMM for budget mix across online/offline; together they form a robust, privacy-resilient stack. (IAB guide; Robyn). (IAB)
Evidence suggests structured PSPs can reduce time-to-therapy and improve adherence — two levers with direct commercial impact. (IQVIA: 17% TtT reduction example). (IQVIA)
Days 0–30: data/consent audit, UTM & taxonomy standards, connect priority systems.
Days 31–60: identity stitching (NPI/HCO + privacy-safe keys), launch first next-best-action journeys, start a holdout test.
Days 61–90: import offline conversions (hub events), extend to field triggers, reallocate budget to measured winners.
Use event time stamps from EHR/hub/POC systems (benefits verification, PA approval, first fill), joined in a privacy-safe way to compute medians and distribution shifts by cohort. Pair this with qualitative root-cause tags to guide interventions.